Serving Patients Through Cellular and Biologic Innovation
We are a clinical-stage biotech company dedicated to the discovery, development, and commercialization of advanced therapeutics that leverage the biological properties of human perinatal tissues and cells. All our efforts are made to support patients and help fulfill their unmet clinical needs.
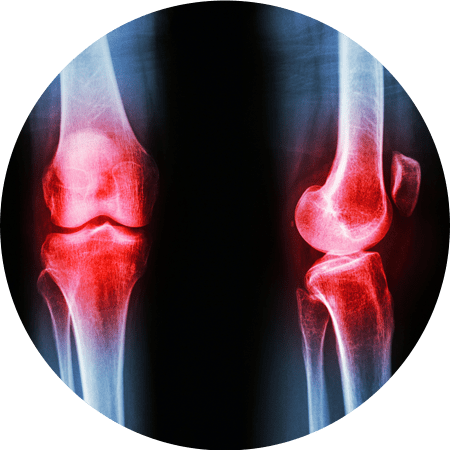
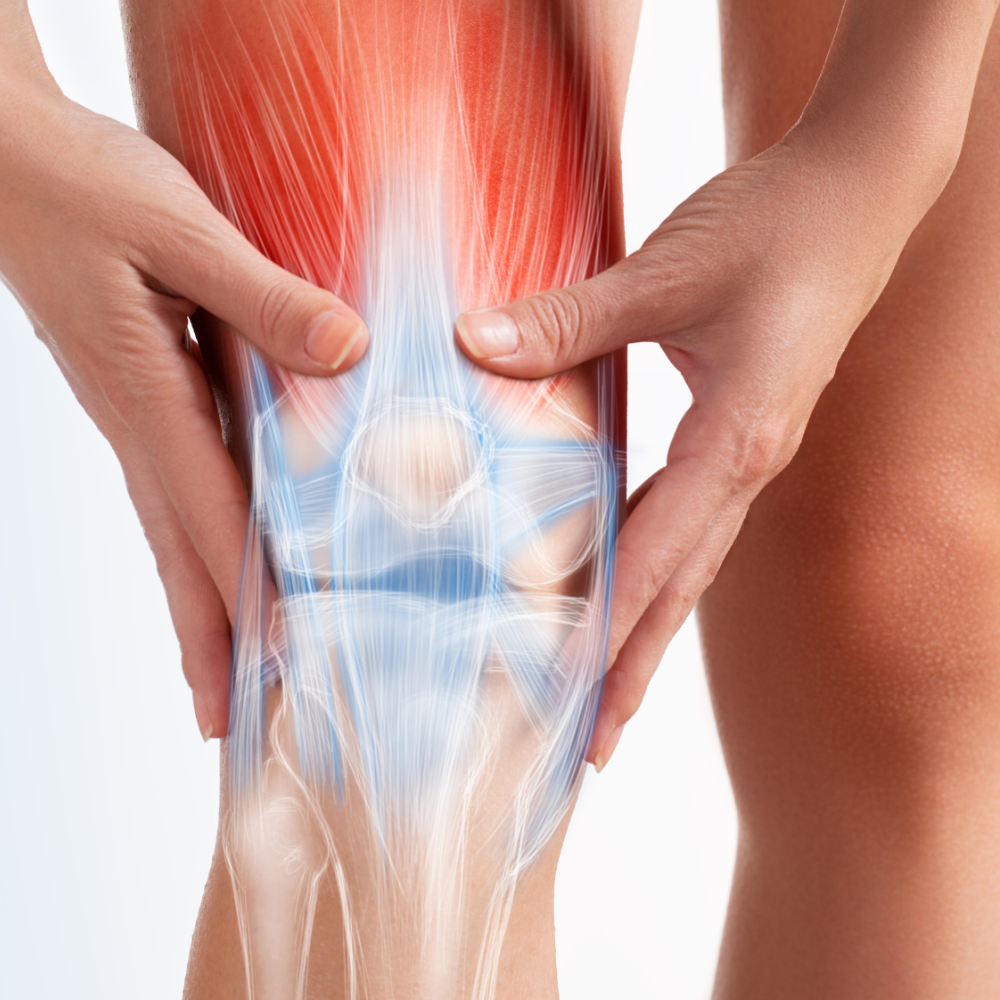
Our leading asset, Signature Cord Prime™, is focused on treating the signs and symptoms of osteoarthritis of the knee. In addition, Arugula is working towards developing and commercializing other novel therapies treating chronic inflammatory and degenerative diseases.
Mesenchymal Stem Cells
Umbilical Cord Mesenchymal Stem Cells (UC-MSC) or Medicinal Signaling Cells, are cells derived from the umbilical cord which are increasingly recognized as having superior therapeutic potential and robust manufacturing behavior compared to MSCs from other sources. At Arugula Biosciences we have compiled may years of clinical, research, regulatory, and business experience to pursue clinical approval UC-MSCs in the US. Currently in R&D, we are planning on pursuing FDA clinical trials soon.
Signature Cord PrimeTM
Flowable Umbilical Cord Tissue Allograft
Signature Cord PrimeTM is an injectable human Umbilical Cord Allograft under clinical trial (FDA Phase 1) to test the clinical effect on patients with symptomatic knee osteoarthritis (NCT05234489). Signature Cord PrimeTM product is a cGMP human umbilical cord allograft product standardized and characterized for safety, potency, purity, and identity.
Signature Cord PrimeTM is an injectable human Umbilical Cord Allograft under clinical trial (FDA Phase 1) to test the clinical effect on patients with symptomatic knee osteoarthritis (NCT05234489). Signature Cord PrimeTM product is a cGMP human umbilical cord allograft product standardized and characterized for safety, potency, purity, and identity.
Mesenchymal Stem Cells
Umbilical Cord Mesenchymal Stem Cells (UC-MSC)
Umbilical Cord Mesenchymal Stem Cells or Medicinal Signaling Cells, are cells derived from the umbilical cord which are increasingly recognized as having superior therapeutic potential and robust manufacturing behavior compared to MSCs from other sources. At Arugula Biosciences we have compiled may years of clinical, research, regulatory, and business experience to pursue clinical approval UC-MSCs in the US. Currently in R&D, we are planning on pursuing FDA clinical trials soon.

Acellular Technologies
Secretome
At Arugula Sciences, we have a portfolio of acellular products poised to become the new generation of drugs with potent immunomodulation and regeneration potential. We are currently developing acellular drugs with transforming clinical applications soon to be deployed in clinical trials in the US.
Signature Cord PrimeTM
Flowable Umbilical Cord Tissue Allograft
Signature Cord PrimeTM is an injectable human Umbilical Cord Allograft under clinical trial (FDA Phase 1) to test the clinical effect on patients with symptomatic knee osteoarthritis (NCT05234489). Signature Cord PrimeTM product is a cGMP human umbilical cord allograft product standardized and characterized for safety, potency, purity, and identity.

Arugula News
January 31, 2023
BusinessWire
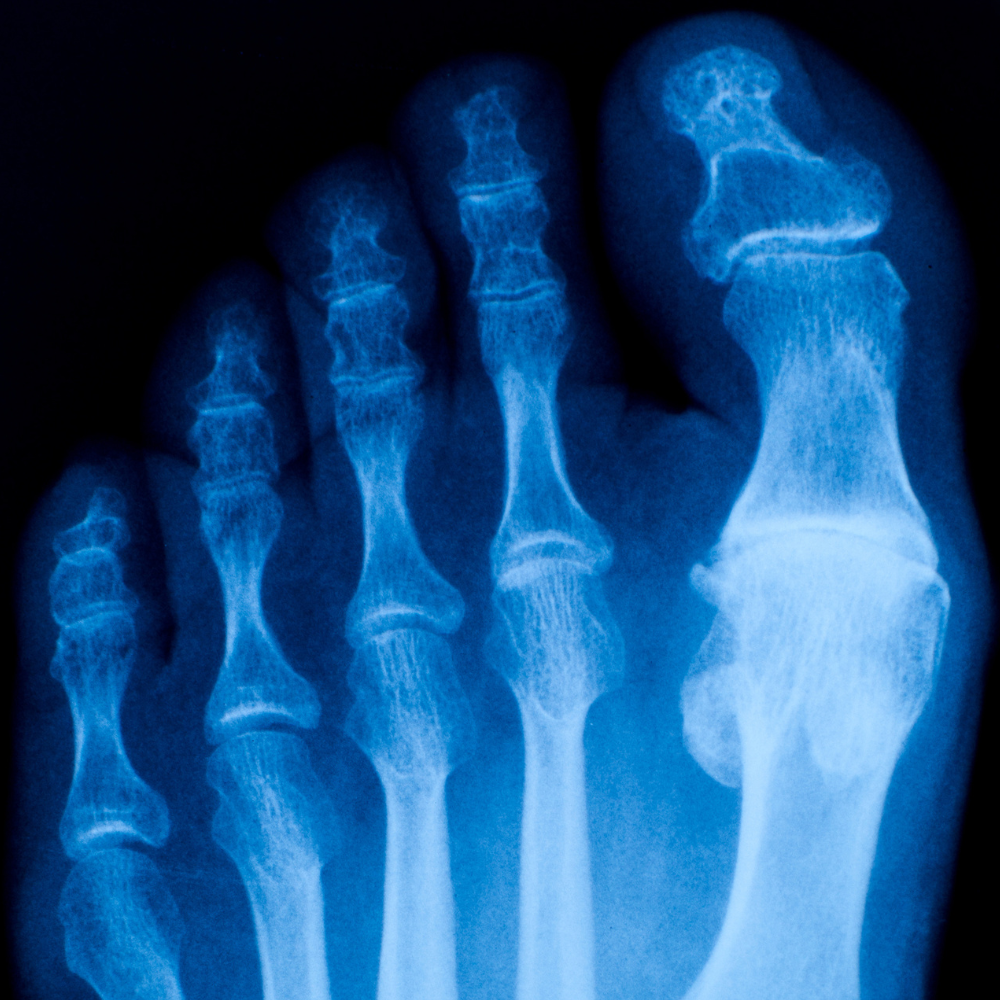
Arugula Sciences announces FDA Approval to Proceed with Phase 1 Study of Treatment of Big Toe Osteoarthritis
January 4, 2023
BusinessWire
Our Team

DR. RAMON CORONADO
CHIEF SCIENTIFIC OFFICER
- Academia
- About
- Experience
Ph.D. Biomedical Engineering at UT San Antonio/ UT Health San Antonio; Technology Entrepreneurship and Management Graduate at UT San Antonio
Joined in 2021 and leads the R&D, Clinical, and Regulatory efforts of Arugula Sciences. As CSO is responsible for overseeing all aspects of the company’s scientific endeavors, including developing new products or services, conducting clinical research, and ensuring that quality standards are met.
Over 10 years of experience in cellular and regenerative medicine research and extensive experience in start-up companies focused on biomedical technologies. Dr. Coronado has filed multiple patent applications in biotech and has founded several biomedical companies in an effort to challenge the status quo of medicine by bringing better medical solutions to patients.

DR. DIANA CASTRO
ADVISORY BOARD MEMBER
- Academia
- About
- Experience
Dr. Castro received her medical degree at the Pontificia Universidad Javeriana in Bogota, Colombia. She did her residency in General Pediatrics at SUNY Downstate, residency in child neurology and fellowship in neuromuscular medicine at the University of Texas Southwestern in Dallas.
After more than ten years, Dr. Castro left academia with the objective of creating a non-profit private practice and research institute for neuromuscular conditions.
Dr. Castro, a board-certified neurologist and neuromuscular physician, is a pioneer in research and management of patients with Spinal Muscular Atrophy (SMA), Duchenne Muscular Dystrophy (DMD), Myasthenia Gravis, and Acquired Neuropathies, like Chronic Inflammatory Demyelinating Polyneuropathy (CIDP).

